An Atom With 2 Electrons in Its Outermost Shell
Where n is the number of shell lives maximum electrons that can be placed in any shell. Valance Electron The electrons in an incomplete outermost orbit are called valance electrons DESCRIPTION.
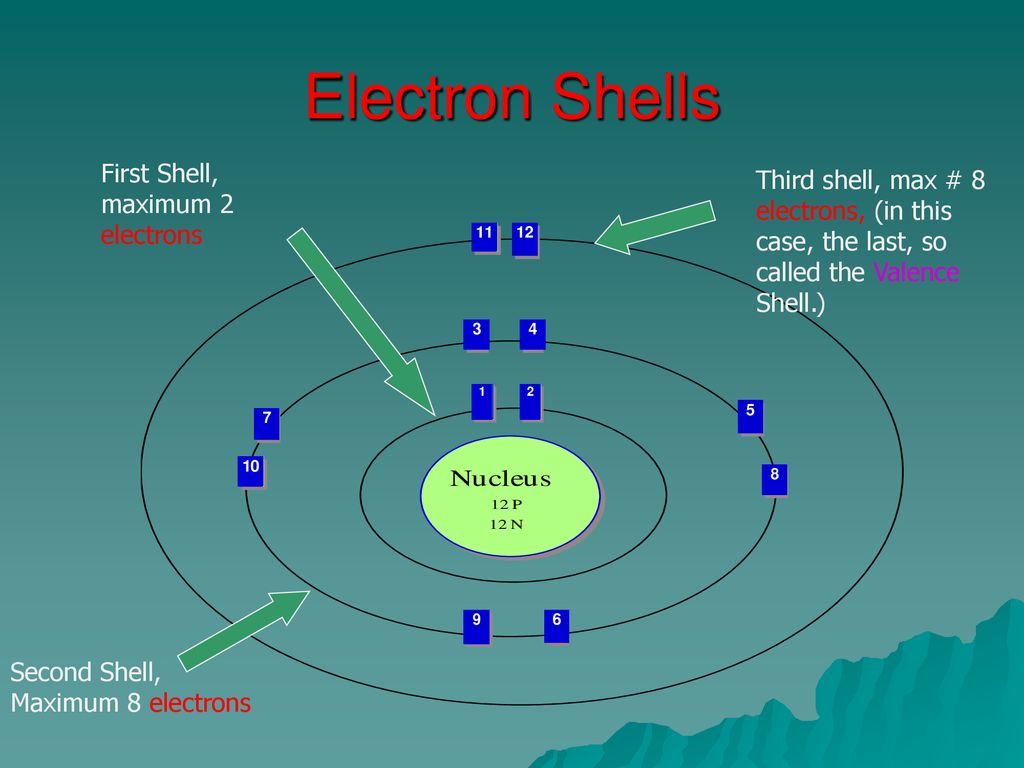
Electron Shells First Shell Maximum 2 Electrons Ppt Download
Of the inert elements Helium atom has 2 electrons in the outermost shell and the atoms of other elements have 8 electrons in.

. The electrons present in the outermost shell of an atom are called valence electrons. For distribution of electrons in the shells 2n 2 rule is used. Of the inert elements Helium atom has 2 electrons in the outermost shell and the atoms of other.
The electrons present in the outermost shell of an atom are called valence electrons. The next most-complex atom is helium which has two protons in its nucleus and two orbiting electrons. If an atoms outermost shell is completely filled they are inert or least reactive and their combining capacity or valency is zero.
Because the closest shell is filled the third electron goes into the next higher shell. The first orbital an s. An atoms nth electron shell can accommodate 2n 2 electrons for example the first shell can accommodate 2 electrons the.
While the outermost electron in an atom or ion with only one electron in the outermost shell orbits a core with effective charge Z k where k is the total number of electrons in the inner shells. The electrons of an atom are typically divided into two categories. Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy levels.
Remember the valence electrons are found in the outermost shell. This difference greatly influences the role of the two types of electrons in a chemical reaction. Of the inert elements Helium atom has 2 electrons in the outermost shell and the atoms of other elements have 8 electrons in their outermost shell.
For example the lithium atom has two electrons in the lowest 1s orbit and these orbit at Z 2. If an atoms outermost shell is completely filled they are inert or least reactive and their combining capacity or valency is zero. These electrons fill the two available states in the lowest shell producing what is called a filled shell.
The shell model was able to qualitatively explain many of the mysterious properties of atoms. Greg RobsonCC BY 20. Build an Atom - PhET Interactive Simulations.
The filling of the electron shells depends on their orbital. Valency is the combining capacity of an atom of an element. Valence and core electrons.
The next atom is lithium with three electrons. Pauli realized that the Zeeman effect must be due only to the outermost electrons of the atom and was able to reproduce Stoners shell structure but with the correct structure of subshells by his inclusion of a fourth quantum number and his exclusion. If an atoms outermost shell is completely filled they are inert or least reactive and their combining capacity or valency is zero.
Lithium is the first element in which an additional electron shell is added. Valance electrons are less tightly bound to the atom than those closer to the nucleus. The electrons present in the outermost shell of an atom are called valence electrons.

Solved An Atom Has 2 Electrons In Its Outermost Shell Chegg Com

An Element X Has 2 Electrons In Its M Shell It Forms A Bond With An Element Y Which Has 7 Electrons In Its Third Orbit Write The Formula Of The Compound

How Many Valence Electrons Does Carbon Have Perfect Atom Electron Configuration Electrons Covalent Bonding

Lesson Explainer Electron Energy Levels Nagwa

How Many Valence Electrons Does Carbon Have Perfect Atom Electrons Covalent Bonding Nomenclature Chemistry
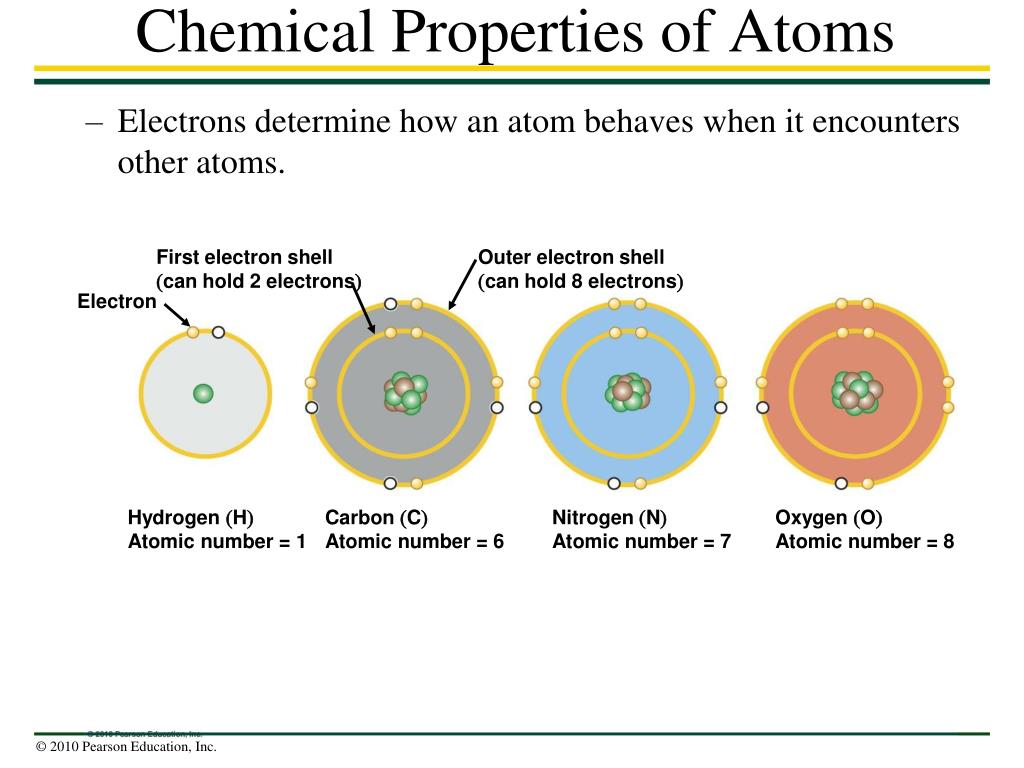
Ppt Chemical Properties Of Atoms Powerpoint Presentation Free Download Id 1418316

Electron Shell Images Stock Photos Vectors Shutterstock
What Are The Electrons Found In An Atom S Outer Shell Often Called Quora

An Element Has 2 Electrons In Its N Shell I What Is Its Atomic Number Ii State Its Position In Periodic Table Iii Is It Metal Or Non Metal Iv State The Name

Dot And Cross Diagram Of Magnesium Chloride Chemistry Chemical Bond Secondary School

Bohr Model Energy Level Of Electrons Cartoon Energy Level Energy Electrons

Cana Modz Chemistry Classroom Science Chemistry Teaching Chemistry

Task 1 Glossary Prezentaciya Na Slide Share Ru

The Orbits Of An Atom Electrons Energy Level Energy
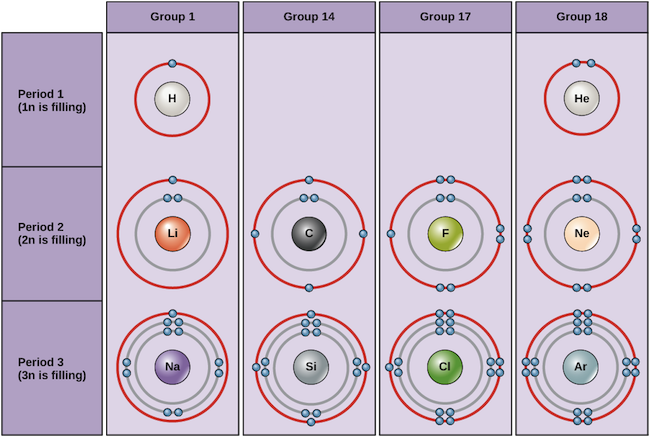
The Periodic Table Electron Shells And Orbitals Article Khan Academy

Electron Shells A Atomic Number Number Of Electrons Electrons Are Placed In Shells According To Rules 1 The 1st Shell Can Hold Up To Two Electrons Ppt Download

How Many Valence Electrons Does Carbon Have Perfect Atom Electrons Electron Configuration Business Plan Template

Atomic Electron Shells Animated Presentation Youtube Nursing School Notes Teaching Science Homeschool Science
Comments
Post a Comment